What Parameters Do Infant Incubator Analyzers Measure?
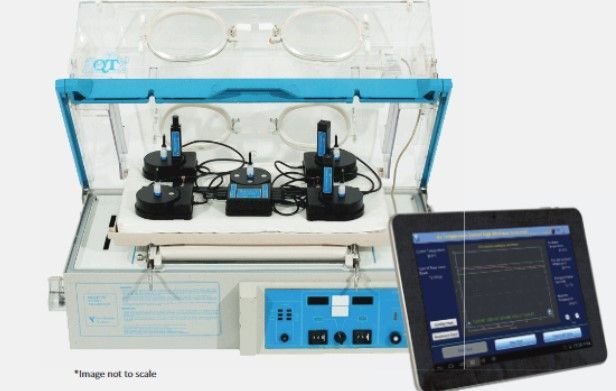
Datrend Systems next generation Incubator Tester, vPad‐IN, is based on our revolutionary Vision‐Pad Technology TM.
Utilizing the Vision‐Pad platform, parameters are sensed inside the incubator environment and results are transmitted wirelessly to a 10" Android tablet for analysis and display.
Using wireless control, test parameters and test protocols can be modified without disturbing the equilibrium of the heated environment, thereby minimizing test times.
What are infant incubator analyzers?
Incubators are devices that provide the necessary environment for an incubation process. The process includes monitoring and caring for the baby.
The incubator analyzer is an analyzing tool that displays information about an incubator's performance. It is important to know how close values are to the true values for accurate treatment.
Periodic tests and controls are needed to guarantee accuracy in medical devices, tests, and systems. This will help you reduce risks by preventing incorrect measurement.
What parameters do infant incubator analyzers measure?
1. Temperature
Measurement Range: 0 °C to 50 °C
Accuracy: T1+/‐ 0.05 °C
Resolution: 0.01 °C
Convection: 5 “puck” sensors (T1 ‐ T5) ‐ radiant warmer
Air Convection: 5 sensors (alternate “pos” T1 ‐ T5) ‐ incubator 1 Mattress Temperature Sensor
Skin Sensor 2 : Sensor “oven” with thermal pad contact 28 °C to 40 °C in 2 °C increments
Resolution: 0.01 °C
Accuracy: +/‐ 0.05 °C
2. Airflow
Measurement Range: 0.1 to 1 m/s
Accuracy: +/‐ 0.1 m/s at 50% RH +/‐ 15%
Resolution : 0.01 m/s
3. Sound Level
Measurement Range: 30 dBA to 100 dBA, Type III
Accuracy: +/‐ 3 dBA
Resolution: 0.1 dBA
4. Relative Humidity
Measurement Range: 0 ‐ 100% Non‐condensing
Accuracy: +/‐ 3% to 100% RH
Resolution: 0.1% RH
5. Data Retention
Capacity: up to 48 hours per test
Sample rate: 1/min or 1/sec, automatic, parameter dependent
Datrend Systems Incubator Tester Specification
Incubator Testing Specifications
• Measures temperature at 6 points
as per IEC 60601‐2‐19 (sub‐clause 201.12.1.101\102)
• Measures airflow at 4 points in the enclosure
per IEC 60601‐2‐19 (sub‐clause 201.12.1.111)
• Measures sound level inside the incubator
per IEC 60601‐2‐19 (sub‐clause 201.9.6.2.1.101)
• Measures sound level inside the incubator during alarm
per IEC 60601‐2‐19 (sub‐clause 201.9.6.2.1.103)
• Measures sound level outside the incubator
per IEC 60601‐2‐19 (sub‐clause 201.9.6.2.1.102)
• Measures incubator temperature at center point (A) of incubator
as per IEC 60601‐2‐19 (subclause 201.12.1.102)
• Measures average incubator temperature (AIT)
per IEC 60601‐2‐19 (sub‐clause 201.3.202)
• Measures incubator temperature (IT)
per IEC 60601‐2‐19 (sub‐clause 201.3.207)
• Measures average temperature (AT)
per IEC 60601‐2‐19 (subclause 201.3.203)
• Measures average temperature at 4 points
per IEC 60601‐2‐19 (sub‐clause 201.12.1.102)
• Measures skin temperature sensor accuracy
per IEC 60601‐2‐19 (sub‐clause 201.12.1.103) 1
• Confirms skin temperature sensor control of incubator
per IEC 60601‐2‐19 (sub‐clause 201.12.1.104)
• Confirms independent incubator temperature sensor accuracy
per IEC 60601‐2‐19 (sub‐clause 201.12.1.105)
• Measures difference between average temperature and control temperature
per IEC 60601‐2‐19 (sub‐clause 201.12.1.106)
• Measures actual warm‐up time compared to operating manual
per IEC 60601‐2‐19 (sub‐clause 201.12.1.107)
• Measures overshoot and recovery to steady state
per IEC 60601‐2‐19 (sub‐clause 201.12.1.108)
• Confirms humidity reading
per IEC 60601‐2‐19 (sub‐clause 201.12.1.109)
Radiant Warmer Testing Specifications
• Measures skin temperature sensor accuracy
per IEC 60601‐2‐21 (sub‐clause 201.12.1.101) 1
• Measures average warmer temperature at 4 points
per IEC 60601‐2‐21 (sub‐clause 201.12.1.102)
• Measures average warmer temperature at center point (A) of incubator
as per IEC 60601‐2‐21(sub‐clause 201.12.1.102)
• Confirms skin temperature sensor control of warmer
per IEC 60601‐2‐21 (sub‐clause 201.12.1.103)
• Confirms airflow is less than 0.1 m/s
per IEC 60601‐2‐21 (subclause 201.12.1.102)
• Measures sound level on the mattress during alarm
per IEC 60601‐2‐21 (sub‐clause 201.9.6.2.1.101)
• Measures sound level in front of the warmer
per IEC 60601‐2‐21 (sub‐clause 201.9.6.2.1.101)
Transport Incubators
Testing carried out in accordance with IEC601‐2‐20
Utility App
Enables ad‐hoc testing of airflow, humidity, sound & temperature 1 Sensor accuracy heater is an optional accessory.
All specifications subject to change without notice.
In Conclusion
An incubator is a device that artificially maintains the body temperature of premature babies. The parameters that an incubator analyzer measures are the temperature, humidity, and air flow.
Datrend Systems’ next generation incubator tester is based on our revolutionary Vision‐Pad technology. This product will greatly help in reducing risks by preventing incorrect measurement of the infant incubator.
Learn more about biomedical testing equipment from Smart Instruments.






